Electron configuration
An electron configuration is the arrangement of electrons within an atom. The electron configuration describes where the electrons are inside orbitals. The structure of the Periodic table of elements is partly based on electron configuration.
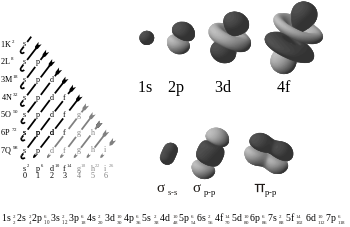
There are four kinds of electron configurations: s, p, d, and f orbitals. Each orbital can house a maximum of 2 electrons. S orbitals are roughly sphere-shaped, p orbitals are polar and shaped like a dumbbell, d orbitals are usually shaped like a four-leaf clover, and f orbitals form a mathematically complex shape. Orbitals can have different energy levels. For example 1s is a low energy sphere-shaped orbital while 3s is a higher energy sphere-shaped orbital. An atom can have more than one orbital; in fact, all except hydrogen do. Similarly, atoms can have more than one of each kind of orbital.
The electron configurations fill up with electrons in an unchanging order. They describe how the electrons behave and orbit the nucleus.