Hydron
cationic form of atomic hydrogen (symbol H⁺), informally called "proton"
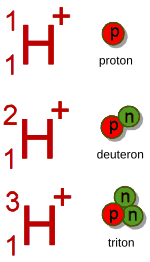
In chemistry a proton is a positively charged hydrogen ion. According to IUPAC, the name hydron should be used for such a particle.[1] In this sense, a hydron can be a proton in the sense of physics, a deuteron or a triton. A deuteron is the ion of deuterium, a triton that of tritium. (So chemists use the term hydron because it covers all three hydrogen ion isotopes.) In 1923, Johannes Nicolaus Brønsted and Thomas Lowry used hydrons to describe acids and bases. They defined acids as proton donors and bases as proton acceptors.
References change
- ↑ Bunnett, J. F.; Jones, R. A. Y. (1968). "Names for hydrogen atoms, ions, and groups, and for reactions involving them (Recommendations 1988)" (PDF). Pure Appl. Chem. 60 (7): 1115–6. doi:10.1351/pac198860071115. S2CID 96121851.