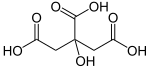
Citric acid
weak organic acid
(Redirected from Citrate)
Citric acid is a weak organic acid. It can be found in citrus fruits. It is used by organisms for Krebs cycle. It acts like a preservative when added to food. It is also used to add a sour (acidic) taste to foods and soft drinks. In the European Union it is known as E 330, as a food additive.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Hydroxypropane-1,2,3-tricarboxylic acid | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.973 | ||
| EC Number |
| ||
| E number | E330 (antioxidants, ...) | ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C6H8O7 | |||
| Molar mass | 192.123 g/mol (anhydrous), 210.038 g/mol (monohydrate)[1] | ||
| Appearance | Crystalline white solid | ||
| Odor | Odorless | ||
| Density | 1.665 g/cm3 (anhydrous) 1.542 g/cm3 (18 °C, monohydrate) | ||
| Melting point | 156 °C (313 °F; 429 K) | ||
| Boiling point | 310 °C (590 °F; 583 K) decomposes from 175 °C[2] | ||
| 117.43 g/100 mL (10 °C) 147.76 g/100 mL (20 °C) 180.89 g/100 mL (30 °C) 220.19 g/100 mL (40 °C) 382.48 g/100 mL (80 °C) 547.79 g/100 mL (100 °C)[3] | |||
| Solubility | soluble in acetone, alcohol, ether, ethyl acetate, DMSO insoluble in C 6H 6, CHCl3, CS2, toluene[2] | ||
| Solubility in ethanol | 62 g/100 g (25 °C)[2] | ||
| Solubility in amyl acetate | 4.41 g/100 g (25 °C)[2] | ||
| Solubility in diethyl ether | 1.05 g/100 g (25 °C)[2] | ||
| Solubility in 1,4-Dioxane | 35.9 g/100 g (25 °C)[2] | ||
| log P | −1.64 | ||
| Acidity (pKa) | pKa1 = 3.13 pKa2 = 4.76 pKa3 = 6.39,[4] 6.40[5] | ||
Refractive index (nD)
|
1.493–1.509 (20 °C)[3] 1.46 (150 °C)[2] | ||
| Viscosity | 6.5 cP (50% aq. sol.)[3] | ||
| Structure | |||
| Monoclinic | |||
| Thermochemistry | |||
| Std enthalpy of formation ΔfH |
−1548.8 kJ/mol[3] | ||
| Std enthalpy of combustion ΔcH |
−1960.6 kJ/mol[6] −1972.34 kJ/mol (monohydrate)[3] | ||
| Standard molar entropy S |
252.1 J/(mol·K)[6] | ||
| Specific heat capacity, C | 226.51 J/(mol·K) (26.85 °C)[6] | ||
| Pharmacology | |||
| A09AB04 (WHO) | |||
| Hazards | |||
| Main hazards | skin and eye irritant | ||
| NFPA 704 |
| ||
| Explosive limits | 8% | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||

Carl Wilhelm Scheele was the first who could extract citric acid from lemons, in 1782. The substance was probably known to alchemists, perhaps with a different name. The Arabian alchemist Geber is said to have discovered citric acid in the 9th century. Citric Acid contains 6 Carbon atoms, 8 Hydrogen atoms and 7 Oxygen atoms. Its chemical formula is C6H8O7.
Main uses
change- As a water softener
- It is often used in detergents, to avoid the smell of acid, esp. Acetic acid
- As a preserving agent
- Citric acid and its salts prevent blood clotting. Blood donations are kept liquid using citric acid.
References
change- ↑ CID 22230 from PubChem
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "citric acid". chemister.ru. Archived from the original on 2014-11-29. Retrieved 2019-02-05.
- ↑ 3.0 3.1 3.2 3.3 3.4 CID 311 from PubChem
- ↑ "Data for Biochemical Research". ZirChrom Separations, Inc. Retrieved January 11, 2012.
- ↑ "Ionization Constants of Organic Acids". Michigan State University. Retrieved January 11, 2012.
- ↑ 6.0 6.1 6.2 Citric acid in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov (retrieved 2014-06-02)
Other websites
change