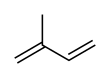
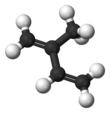
Isoprene
Isoprene is a common organic compound produced by living things. Its full chemical name is 2-methyl-1,3-butadiene, and its formula is CH2=C(CH3)−CH=CH2. Isoprene is an unsaturated hydrocarbon. It is produced by many plants and animals (including humans). Its polymers are the main component of natural rubber.[1][2][3]
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Methylbuta-1,3-diene | |||
| Other names
2-Methyl-1,3-butadiene
Isoprene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.040 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C5H8 | |||
| Molar mass | 68.12 g/mol | ||
| Density | 0.681 g/cm3 | ||
| Melting point | −143.95 °C (−227.11 °F; 129.20 K) | ||
| Boiling point | 34.067 °C (93.321 °F; 307.217 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Natural occurrences
changeIsoprene is produced and emitted by many species of trees (major producers are oaks, poplars, eucalyptus, and some legumes). Yearly production of isoprene emissions by vegetation is around 600 million metric tons, half from tropical broadleaf trees (that is, flowering plants) and the rest mainly from shrubs.[4]
This is about the same as methane emissions. It accounts for about one-third of all hydrocarbons released into the atmosphere. In deciduous forests, isoprene makes up about 80% of hydrocarbon emissions. Microscopic and macroscopic algae also produce isoprene, but much less than trees.[5]
Isoprene emission may help trees use to combat stress.[6] In particular, isoprene does protect against moderate heat stress (around 40 °C). It may also protect plants against large fluctuations in leaf temperature. Isoprene is built into cell membranes and helps membranes keep stable.
Isoprene produces the blue haze which gives the Blue Ridge Mountains their name.
References
change- ↑ Sharkey, Thomas D. (1996). "Isoprene synthesis by plants and animals". Endeavour. 20 (2): 74–78. doi:10.1016/0160-9327(96)10014-4. PMID 8690002.
- ↑ Williams, C. Grenville (1860). "On isoprene and caoutchine". Proceedings of the Royal Society of London. 10: 516–519. doi:10.1098/rspl.1859.0101. S2CID 104233421.
- ↑ M. J. Loadman (2012-12-06). Analysis of Rubber and Rubber-like Polymers. Springer. p. 10. ISBN 9789401144353.
- ↑ Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P. I.; Geron, C. (2006). "Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature)". Atmospheric Chemistry and Physics. 6 (11): 3181–3210. Bibcode:2006ACP.....6.3181G. doi:10.5194/acp-6-3181-2006.
- ↑ Johnston, Antonia; Crombie, Andrew T.; El Khawand, Myriam; Sims, Leanne; Whited, Gregg M.; McGenity, Terry J.; Colin Murrell, J. (September 2017). "Identification and characterisation of isoprene-degrading bacteria in an estuarine environment: Estuarine isoprene-degrading bacteria". Environmental Microbiology. 19 (9): 3526–3537. doi:10.1111/1462-2920.13842. PMC 6849523. PMID 28654185.
- ↑ Sharkey, T. D.; Wiberley, A. E.; Donohue, A. R. (2007). "Isoprene emission from plants: why and how". Annals of Botany. 101 (1): 5–18. doi:10.1093/aob/mcm240. PMC 2701830. PMID 17921528.