Propane
chemical compound, commonly used as a fuel
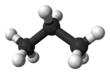
Propane is an organic compound with the chemical formula C
3H
8. It is an alkane with three carbon atoms. It is used in fuels.
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Propane[1] | |||
| Systematic IUPAC name
Tricarbane (never recommended[1]) | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| Beilstein Reference | 1730718 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.753 | ||
| EC Number |
| ||
| E number | E944 (glazing agents, ...) | ||
| Gmelin Reference | 25044 | ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1978 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties[3] | |||
| C3H8 | |||
| Molar mass | 44.10 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Odorless | ||
| Density | 2.0098 kg/m3 (at 0 °C, 101.3 kPa) | ||
| Melting point | −187.7 °C; −305.8 °F; 85.5 K | ||
| Boiling point | −42.25 to −42.04 °C; −44.05 to −43.67 °F; 230.90 to 231.11 K | ||
| 47 mg⋅L−1 (at 0 °C) | |||
| log P | 2.236 | ||
| Vapor pressure | 853.16 kPa (at 21.1 °C (70.0 °F)) | ||
| kH | 15 nmol⋅Pa−1⋅kg−1 | ||
| Conjugate acid | Propanium | ||
| −40.5 × 10−6 cm3/mol | |||
| 0.083 D[2] | |||
| Thermochemistry | |||
| Std enthalpy of formation ΔfH |
−105.2–104.2 kJ⋅mol−1 | ||
| Std enthalpy of combustion ΔcH |
−2.2197–2.2187 MJ⋅mol−1 | ||
| Specific heat capacity, C | 73.60 J⋅K−1⋅mol−1 | ||
| Hazards | |||
| NFPA 704 |
| ||
| Explosive limits | 2.37–9.5% | ||
| U.S. Permissible exposure limit (PEL) |
TWA 1000 ppm (1800 mg/m3)[4] | ||
| Related compounds | |||
| Related {{{label}}} | {{{value}}} | ||
| Related compounds | {{{value}}} | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
It begins to burn very quickly. Its melting temperature is −187.7 °C; its boiling temperature is −42 °C; its density is 1.83 g/l.
Propane is extracted from natural gasoline or from petroleum.
Sources
change- ↑ 1.0 1.1 "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 4. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
Similarly, the retained names 'ethane', 'propane', and 'butane' were never replaced by systematic names 'dicarbane', 'tricarbane', and 'tetracarbane' as recommended for analogues of silane, 'disilane'; phosphane, 'triphosphane'; and sulfane, 'tetrasulfane'.
- ↑ Lide, David R., Jr. (1960). "Microwave Spectrum, Structure, and Dipole Moment of Propane". J. Chem. Phys. 33 (5): 1514–1518. Bibcode:1960JChPh..33.1514L. doi:10.1063/1.1731434.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Record of Propane in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ NIOSH Pocket Guide to Chemical Hazards. "#0524". National Institute for Occupational Safety and Health (NIOSH).
- ↑ GOV, NOAA Office of Response and Restoration, US. "PROPANE – CAMEO Chemicals – NOAA". cameochemicals.noaa.gov.
{{cite web}}: CS1 maint: multiple names: authors list (link)