Selenium tetrachloride
Selenium tetrachloride, also selenious chloride, selenous chloride, or selenium(IV) chloride, is a chemical compound. Its chemical formula is SeCl4. It contains selenium in its +4 oxidation state. It also contains chloride ions.
| ||

| ||
| Names | ||
|---|---|---|
| IUPAC name
Selenium tetrachloride
| ||
| Identifiers | ||
3D model (JSmol)
|
||
| ChemSpider | ||
| ECHA InfoCard | 100.030.036 | |
PubChem CID
|
||
| RTECS number |
| |
CompTox Dashboard (EPA)
|
||
| ||
| Properties | ||
| SeCl4 | ||
| Molar mass | 220.771 g/mol | |
| Appearance | white to yellow crystals | |
| Density | 2.6 g/cm³, solid | |
| Melting point | sublimes at 191.4°C[1] | |
| decomposes in water | ||
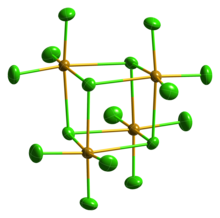
| Structure | ||
| Monoclinic, mS80 | ||
| C12/c1, No. 15 | ||
| Seesaw (gas phase) | ||
| Hazards | ||
| EU classification | Toxic (T), Dangerous for the environment (N) | |
| NFPA 704 |
| |
| R-phrases | R23/25, R33, R50/53 | |
| S-phrases | S20/21, S28, S45, S60, S61[2] | |
| Flash point | non-flammable | |
| Related compounds | ||
| Other anions | {{{value}}} | |
| Other cations | {{{value}}} | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | ||
| Infobox references | ||
Properties
changeSelenium tetrachloride is a yellow or white solid. It evaporates easily. It reacts with water to make hydrochloric acid and selenous acid. It reacts with selenium dioxide to make selenium oxychloride, a mixture of selenium dioxide and selenium tetrachloride bonded together.
Preparation
changeIt is made by heating a mixture of selenium and chlorine. The selenium tetrachloride escapes as a gas. This can be used to purify selenium. Impure selenium can be placed in the flask, reacted with chlorine. Only selenium tetrachloride escapes. This is reduced to selenium again, making pure selenium.
Uses
changeIt is used to purify selenium. It is used to make other selenium compounds.
Related pages
changeReferences
change- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, FL: CRC Press. p. 487. ISBN 0849305942. Retrieved 2008-07-02.
- ↑ "323527 Selenium tetrachloride". Sigma-Aldrich. Retrieved 2008-07-02.[permanent dead link]
