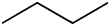Butane
chemical compound
Butane is an organic compound with the chemical formula C
4H
10. It is an alkane with four carbon atoms. It is used as a fuel (sometimes with propane) and in aerosol cans.
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Butane[3] | |||
| Systematic IUPAC name
Tetracarbane (never recommended[3]) | |||
| Other names | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| Beilstein Reference | 969129 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.136 | ||
| EC Number |
| ||
| E number | E943a (glazing agents, ...) | ||
| Gmelin Reference | 1148 | ||
| KEGG | |||
| MeSH | butane | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1011 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C4H10 | |||
| Molar mass | 58.12 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Gasoline-like or natural gas-like[1] | ||
| Density | 2.48 kg/m3 (at 15 °C (59 °F)) | ||
| Melting point | −140 to −134 °C; −220 to −209 °F; 133 to 139 K | ||
| Boiling point | −1 to 1 °C; 30 to 34 °F; 272 to 274 K | ||
| 61 mg L−1 (at 20 °C (68 °F)) | |||
| log P | 2.745 | ||
| Vapor pressure | ~170 kPa at 283 K [4] | ||
| kH | 11 nmol Pa−1 kg−1 | ||
| Conjugate acid | Butanium | ||
| -57.4·10−6 cm3/mol | |||
| Thermochemistry | |||
| Std enthalpy of formation ΔfH |
−126.3–−124.9 kJ mol−1 | ||
| Std enthalpy of combustion ΔcH |
−2.8781–−2.8769 MJ mol−1 | ||
| Specific heat capacity, C | 98.49 J K−1 mol−1 | ||
| Hazards | |||
| NFPA 704 |
| ||
| Explosive limits | 1.8–8.4% | ||
| U.S. Permissible exposure limit (PEL) |
none[1] | ||
| Related compounds | |||
| Related {{{label}}} | {{{value}}} | ||
| Related compounds | {{{value}}} | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||

Uses of Butane
changeButane is sold in canisters, for cooking and camping. It is also used as fuel in cigarette lighters, and as propellant in aerosol sprays or deodorants. Some kinds of Butane are used in refrigerators.
Dangers
changeButane can be hazardous. Inhalation can lead to death by asphyxiation due to displacement of oxygen in the lungs. Contact with the skin can lead to frostbite. If the gas is mixed with air and ignighted it is prone to explode like many other fuels that are volatile
References
change- ↑ 1.0 1.1 1.2 NIOSH Pocket Guide to Chemical Hazards. "#0068". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Hofmann, August Wilhelm Von (1 January 1867). "I. On the action of trichloride of phosphorus on the salts of the aromatic monamines". Proceedings of the Royal Society of London. 15: 54–62. doi:10.1098/rspl.1866.0018. S2CID 98496840. Retrieved 20 September 2018 – via rspl.royalsocietypublishing.org.
- ↑ 3.0 3.1 "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 4. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
Similarly, the retained names 'ethane', 'propane', and 'butane' were never replaced by systematic names 'dicarbane', 'tricarbane', and 'tetracarbane' as recommended for analogues of silane, 'disilane'; phosphane, 'triphosphane'; and sulfane, 'tetrasulfane'.
- ↑ W. B. Kay (1940). "Pressure-Volume-Temperature Relations for n-Butane". Industrial & Engineering Chemistry. 32 (3): 358–360. doi:10.1021/ie50363a016.
Other websites
change- International Chemical Safety Card 0232
- NIOSH Pocket Guide to Chemical Hazards
- n-Butane Archived 2008-09-25 at the Wayback Machine Molecule of the Month
- Molview from bluerhinos.co.uk Archived 2008-05-08 at the Wayback Machine See Butane in 3D
- Computational Chemistry Wiki Archived 2009-04-30 at the Wayback Machine
- World LP Gas Association (WLPGA)
- LP Gas Association: Propane and Butane in the UK Archived 2008-04-13 at the Wayback Machine
- Global BioSciences In-Situ Bioremediation utilizing Butane
- Butane Viscosity as function of temperature and pressure