Carbonate
salt or ester of carbonic acid
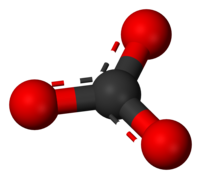
A carbonate is a chemical compound that has the carbonate ion, CO2−
3. This ion is made of carbon and oxygen. The name may also mean an ester of carbonic acid, an organic compound containing the carbonate group C(=O)(O–)2 of carbon and oxygen. They have a valency of 1.
| |
| Names | |
|---|---|
| Systematic IUPAC name
Carbonate | |
| Identifiers | |
| Properties | |
| CO2− 3 | |
| Molar mass | 60.01 g mol-1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
When added to an acid, a carbonate will produce carbon dioxide, water and a chemical salt. Sedimentary rocks containing calcite and other carbonates are plentiful in the earth.
Related pages
change