Cobalt(II) chloride
Cobalt(II) chloride, also known as cobaltous chloride and cobalt dichloride, is a chemical compound. It contains cobalt in its +2 oxidation state. Its chemical formula is CoCl2. It contains cobalt and chloride ions.
| |||
 Structure of anhydrous compound
| |||
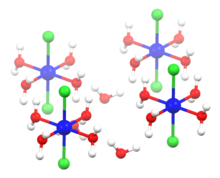 Structure of hexahydrate
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Cobalt(II) chloride
| |||
| Other names | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.718 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII |
| ||
| UN number | 3288 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| CoCl2 | |||
| Molar mass | 129.839 g/mol (anhydrous) 165.87 g/mol (dihydrate) 237.93 g/mol (hexahydrate) | ||
| Appearance | blue crystals (anhydrous) violet-blue (dihydrate) rose red crystals (hexahydrate) | ||
| Density | 3.356 g/cm3 (anhydrous) 2.477 g/cm3 (dihydrate) 1.924 g/cm3 (hexahydrate) | ||
| Melting point | 726 °C (1,339 °F; 999 K) ±2 (anhydrous) 140 °C (monohydrate) 100 °C (dihydrate) 86 °C (hexahydrate) | ||
| Boiling point | 1,049 °C (1,920 °F; 1,322 K) | ||
| 43.6 g/100 mL (0 °C) 45 g/100 mL (7 °C) 52.9 g/100 mL (20 °C) 105 g/100 mL (96 °C) | |||
| Solubility | 38.5 g/100 mL (methanol) 8.6 g/100 mL (acetone) soluble in ethanol, pyridine, glycerol | ||
| +12,660·10−6 cm3/mol | |||
| Structure | |||
| CdCl2 structure | |||
| hexagonal (anhydrous) monoclinic (dihydrate) Octahedral (hexahydrate) | |||
| Hazards | |||
| NFPA 704 |
| ||
| Flash point | Non-flammable | ||
| Related compounds | |||
| Other anions | {{{value}}} | ||
| Other cations | {{{value}}} | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||


Properties
changeCobalt(II) chloride is normally found in the red (or pink) form. The red form has water in it. It can be heated to turn it into the blue form, without water. The blue form will absorb water from the air and turn red again.
A different blue form is made when cobalt(II) chloride is reacted with a chemical compound that has chloride in it. Hydrochloric acid works the best, but sodium chloride can be used too. When it is heated, it turns more blue than when it is cooled.
for an example. Cobalt chloride can be used to test for chloride ions in this way.
It can be oxidized to cobalt(III) compounds, although cobalt(III) chloride does not exist. If it is made, it just drops a Cl from CoCl3, making CoCl2 (cobalt(II) chloride) again.
Preparation
changeThe anhydrous (without water) blue form can be made by reacting cobalt with chlorine. The hydrated (with water) red form can be made by reacting cobalt(II) oxide or cobalt(II) hydroxide with hydrochloric acid.
Uses
changeIt is used to place cobalt into other chemical compounds. It can be used to make other cobalt compounds. It is the most common cobalt compound in the lab.
Safety
changeIt is a weak oxidizing agent, too weak to ignite things. Cobalt compounds are toxic in large quantities, like any other transition metal compounds. They are not toxic like lead or mercury compounds, though.
References
change- ↑ "Cobalt muriate, CAS Number: 7646-79-9". www.chemindustry.com. Archived from the original on 28 May 2019. Retrieved 19 April 2018.


