Electron transport chain
An electron transport chain (ETC) is how a cell gets energy from sunlight in photosynthesis. Electron transport chains also occur in reduction/oxidation ("redox") reactions, such as the oxidation of sugars in cellular respiration.
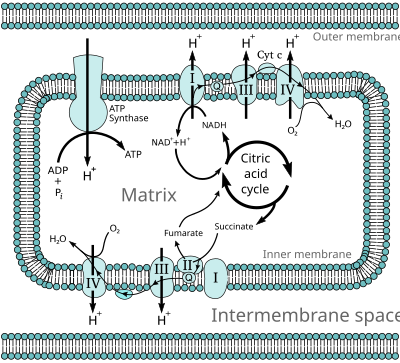

In aerobic respiration, each molecule of glucose leads to about 34 molecules of ATP (Adenosine triphosphate) being produced by the electron transport chain. This is by far the most productive part of respiration.[1]
Background
changeThe electron transport chain consists of a series of redox reactions in which electrons are transferred from a donor molecule to an acceptor molecule. The underlying force driving these reactions is the free energy (energy available to do work) of the reactants and products. Any reaction that decreases the overall free energy of a system will happen.
ATP synthase is an enzyme found among all domains of life. It is powered by a transmembrane proton electrochemical gradient. This is the result of the series of redox reactions.[2] What the electron transport chain does is produce this gradient.[3][4] The free energy is used to drive ATP synthesis.
References
change- ↑ Rich P.R. 2003. The molecular machinery of Keilin's respiratory chain. Biochemical Society Transactions 31 (pt 6): 1095–1105. doi:10.1042/BST0311095 PMID 14641005
- ↑ Karp, Gerald (2008). Cell and molecular biology, 5th ed. Hoboken NJ: Wiley. p. 194. ISBN 978-0-470-04217-5.
- ↑ Murray, Robert K.; et al. (2003). Harper's Illustrated biochemistry, p96. New York, NY: Lange Medical Books/ McGraw Hill. ISBN 0-07-121766-5.
- ↑ Harper's Illustrated Biochemistry explaining the function of the complexes of the transport chain: "Each of the respiratory chain complexes I, II, and IV... acts as a proton pump...creating an electrochemical potential difference across the [mitochondrial inner] membrane". p96