Isolobal principle
The isolobal principle (also known as isolobal analogy) is way to predict bonding properties of organometallic compounds. In organometallic chemistry, it relates the structure of organic ligands that can bind to inorganic molecular fragments.[1] Roald Hoffmann described molecular fragments as isolobal "if the number, symmetry properties, approximate energy and shape of the frontier orbitals and the number of electrons in them are similar – not identical, but similar."[2] One can predict the bonding and reactivity of a lesser-known pieces from that of a better-known pieces if the two molecular fragments have similar frontier orbitals, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). Isolobal compounds are analogs to isoelectronic compounds that share the same number of valence electrons and structure. A graphic representation of isolobal structures, with the isolobal pairs connected through a double-headed arrow with half an orbital below, is found in Figure 1.
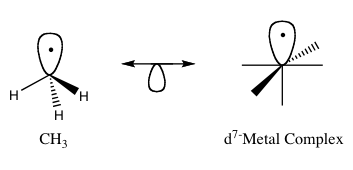
For his work on the isolobal analogy, Hoffmann was awarded the Nobel Prize in Chemistry in 1981, which he shared with Kenichi Fukui.[3] In his Nobel Prize lecture, Hoffmann stressed that the isolobal analogy is a useful, yet simple, model. It does fail in certain instances.[1]
References
change- ↑ 1.0 1.1 Hoffmann, R. (1982). "Building Bridges Between Inorganic and Organic Chemistry (Nobel Lecture)" (PDF). Angew. Chem. Int. Ed. 21 (10): 711–724. doi:10.1002/anie.198207113.
- ↑ In reference 10 of his Nobel Prize acceptance speech, Hoffmann states that the term "isolobal" was introduced in reference 1e, "Elian, M., Chen, M. M.-L., Mingos, D. M. P. and Hoffmann, R., Inorg. Chem., 15, 1148 (1976)", but that the concept is older.
- ↑ "The Nobel Prize in Chemistry 1981: Kenichi Fukui, Roald Hoffmann". nobelprize.org. Retrieved December 22, 2010.