User:MdWikiBot/Methamphetamine
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌmɛθæmˈfɛtəmiːn/ |
| Trade names | Desoxyn, Methedrine, others |
| Synonyms | N-methylamphetamine, N,α-dimethylphenethylamine, desoxyephedrine |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category | |
| Dependence liability | Physical: None; Psychological: High |
| Addiction liability | High |
| Routes of administration | By mouth |
| Drug class | Amphetamine |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | By mouth: 70%[2] IV: 100%[2] |
| Protein binding | Varies widely[3] |
| Metabolism | CYP2D6[1] and FMO3 |
| Onset of action | Rapid[4] |
| Elimination half-life | 5–30 hours |
| Duration of action | 10–20 hours[4] |
| Excretion | Primarily kidney |
| Identifiers | |
| |
| Chemical and physical data | |
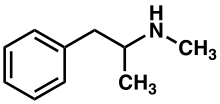
| Formula | C10H15N |
| Molar mass | 149.24 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| Melting point | 170 °C (338 °F) [5] |
| Boiling point | 212 °C (414 °F) at 760 mmHg[5] |
| |
| |
| (verify) | |
Methamphetamine[note 1] is a central nervous system (CNS) stimulant mainly used as a recreational drug and less commonly to treat attention deficit hyperactivity disorder, narcolepsy, and obesity.[11][6] Use for obesity is no longer recommended.[7] When taken by mouth effects begin within 30 minutes and may last for up to 24 hours.[7][6]
Common side effects include high blood pressure, palpitations, elevate mood, trouble sleeping, tremor, diarrhea, and sexual dysfunction.[7] Other side effects may include psychosis, mania, seizures, high body temperature, and tics.[7][12] There is a high risk abuse; though deaths directly from use is rare.[7][6] Use during pregnancy may harm the baby and breastfeeding following using is not recommended.[7][13] It is in the amphetamine family of medication.[7]
Methamphetamine was discovered in 1893 and first manufactured in 1919.[14][6] It is most commonly made in illegal facilities in the United States and Far East.[6] Recreationally it may be swallowed, snorted, injected, or smoked.[6] It is classified as a Schedule II controlled substance.[6] The production, distribution, and possession of methamphetamine is restricted or banned in many countries.[15] In Europe it costs about 17 to 64 Euro per gram for an illegal supply as of 2018.[16] About 27 million people used amphetamines, most methamphetamine, in 2019.[17]
References
change- ↑ Sellers EM, Tyndale RF (2000). "Mimicking gene defects to treat drug dependence". Ann. N. Y. Acad. Sci. 909 (1): 233–246. Bibcode:2000NYASA.909..233S. doi:10.1111/j.1749-6632.2000.tb06685.x. PMID 10911933. S2CID 27787938.
Methamphetamine, a central nervous system stimulant drug, is p-hydroxylated by CYP2D6 to less active p-OH-methamphetamine.
- ↑ 2.0 2.1 Rau T, Ziemniak J, Poulsen D (2015). "The neuroprotective potential of low-dose methamphetamine in preclinical models of stroke and traumatic brain injury". Prog. Neuropsychopharmacol. Biol. Psychiatry. 64: 231–6. doi:10.1016/j.pnpbp.2015.02.013. PMID 25724762.
In humans, the oral bioavailability of methamphetamine is approximately 70% but increases to 100% following intravenous (IV) delivery (Ares-Santos et al., 2013).
- ↑ "Toxicity". Methamphetamine. National Center for Biotechnology Information. Archived from the original on 2015-01-04. Retrieved 2021-10-25.
{{cite encyclopedia}}:|work=ignored (help) - ↑ 4.0 4.1 Riviello RJ (2010). Manual of forensic emergency medicine : a guide for clinicians. Sudbury, Mass.: Jones and Bartlett Publishers. p. 41. ISBN 978-0-7637-4462-5. Archived from the original on 18 March 2017. Retrieved 4 September 2017.
- ↑ 5.0 5.1 "Chemical and Physical Properties". Methamphetamine. National Center for Biotechnology Information. Archived from the original on 2015-01-04. Retrieved 2021-10-25.
{{cite encyclopedia}}:|work=ignored (help) - ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 "Methamphetamine". Drug profiles. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). 8 January 2015. Archived from the original on 15 April 2016. Retrieved 27 November 2018.
The term metamfetamine (the International Non-Proprietary Name: INN) strictly relates to the specific enantiomer (S)-N,α-dimethylbenzeneethanamine.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 "Methamphetamine Monograph for Professionals". Drugs.com. Archived from the original on 6 September 2021. Retrieved 18 November 2021.
- ↑ "Interpreting Urine Drug Tests (UDT)". Archived from the original on 25 October 2023. Retrieved 24 October 2023.
- ↑ "Identification". Methamphetamine. University of Alberta. 8 February 2013. Archived from the original on 28 December 2015. Retrieved 25 October 2021.
{{cite encyclopedia}}:|work=ignored (help) - ↑ "Methedrine (methamphetamine hydrochloride): Uses, Symptoms, Signs and Addiction Treatment". Addictionlibrary.org. Archived from the original on 4 March 2016. Retrieved 16 January 2016.
- ↑ Yu S, Zhu L, Shen Q, Bai X, Di X (March 2015). "Recent advances in methamphetamine neurotoxicity mechanisms and its molecular pathophysiology". Behav. Neurol. 2015: 103969. doi:10.1155/2015/103969. PMC 4377385. PMID 25861156.
In 1971, METH was restricted by US law, although oral METH (Ovation Pharmaceuticals) continues to be used today in the USA as a second-line treatment for a number of medical conditions, including attention deficit hyperactivity disorder (ADHD) and refractory obesity.
- ↑ "Methamphetamine" (PDF). Archived (PDF) from the original on 23 June 2021. Retrieved 19 November 2021.
- ↑ "Methamphetamine". Drugs and Lactation Database (LactMed). National Library of Medicine (US). 2006. Archived from the original on 2 March 2021. Retrieved 19 November 2021.
- ↑ Mack, Avram H.; Brady, Kathleen T.; Frances, Richard J.; Miller, Sheldon I. (12 May 2016). Clinical Textbook of Addictive Disorders, Fourth Edition. Guilford Publications. p. 203. ISBN 978-1-4625-2169-2. Archived from the original on 19 November 2021. Retrieved 18 November 2021.
- ↑ Lilley, Linda Lane; Collins, Shelly Rainforth; Snyder, Julie S. (20 January 2017). Pharmacology for Canadian Health Care Practice. Elsevier Health Sciences. p. 61. ISBN 978-1-77172-022-9. Archived from the original on 19 November 2021. Retrieved 19 November 2021.
- ↑ "Infographic: amphetamine, methamphetamine, seizures, price, purity in the EU, 2018 | www.emcdda.europa.eu". www.emcdda.europa.eu. Archived from the original on 13 November 2021. Retrieved 19 November 2021.
- ↑ World Drug Report 2021 (PDF). United Nations. 2021. ISBN 9789211483611. Archived (PDF) from the original on 3 July 2021. Retrieved 19 November 2021.
Cite error: There are <ref group=note> tags on this page, but the references will not show without a {{reflist|group=note}} template (see the help page).